Science
Key to our innovative approach is our focus on the target ICAM-1 (Intercellular Adhesion Molecule 1) and LFA-1 (Lymphocyte Function-Associated Antigen 1). ICAM-1, found on the surface of cancer cells, acts as a binding site for LFA-1 on T cells, facilitating their recognition and destruction of malignant cells.


Affinity Tuning
Affinity Tuning
By affinity tuning, AffyImmune’s CAR T cells are optimized to kill cancerous cells while avoiding damage to normal cells and tissues.
Noninvasive Monitoring
Noninvasive Monitoring
In vivo tracking of CAR T cells reduces patient burden and improves the standard of care for cancer patients. AffyImmune’s proprietary technology addresses critical challenges such as toxicity, antigen loss, T cell trafficking, and exhaustion.
Focus on ICAM-1
Focus on ICAM-1
ICAM-1, found on the surface of cancer cells, acts as a binding site for LFA-1 on AIC100 CAR T cells, facilitating their recognition and destruction of malignant cells.
Clinical Indications
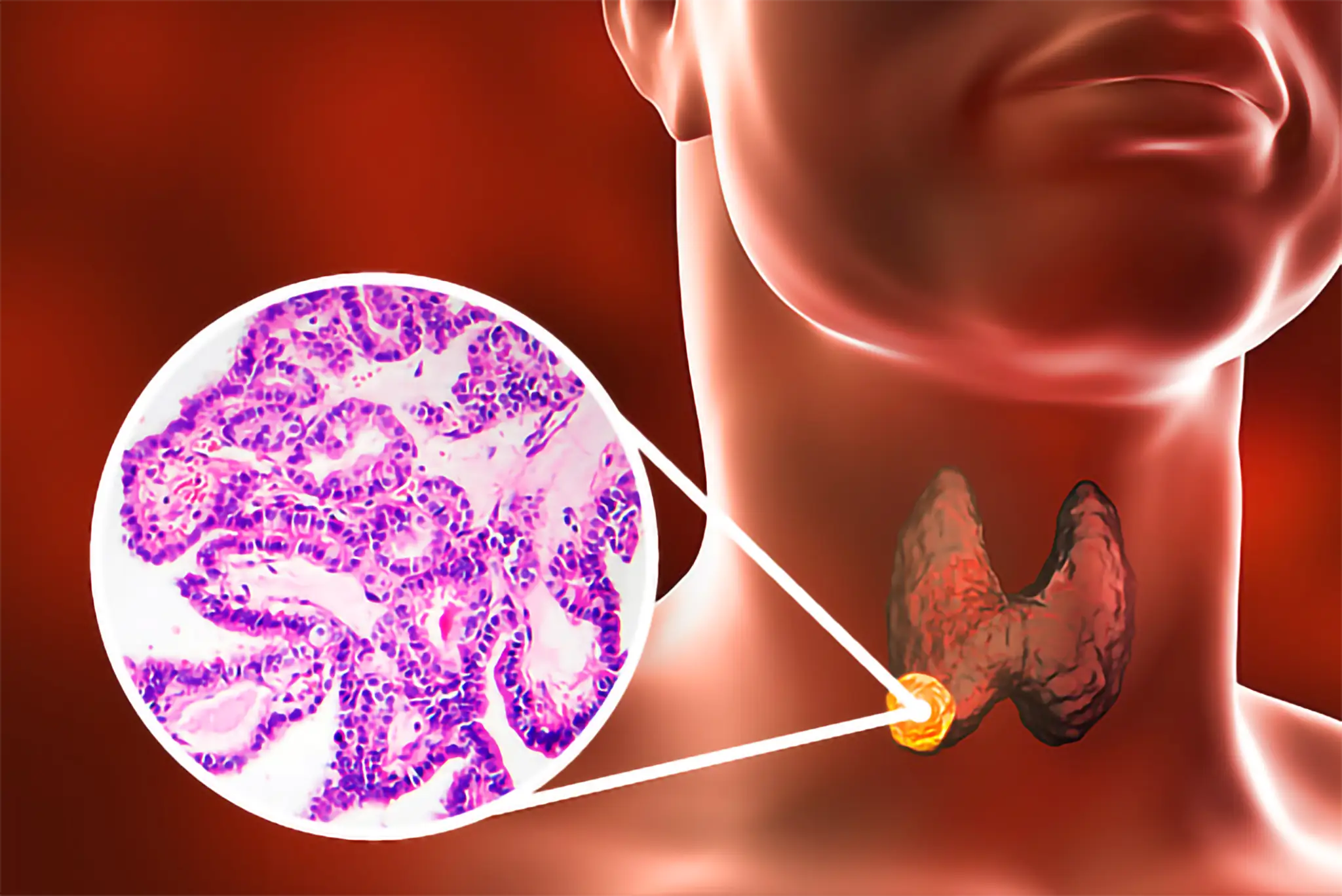
Clinical Indications
AffyImmune has chosen anaplastic and advanced differentiated thyroid cancers as the initial indication for AIC100 and has received Orphan Drug, Fast Track, and Regenerative Medicine Advanced Therapy Designations from the FDA. Non-small cell lung cancer has been selected as the second indication.